Patient Surveys in Blueprint OMS
Are you using patient surveys?
Enhance your overall patient experience by using the following built-in patient survey tools available within Blueprint OMS.
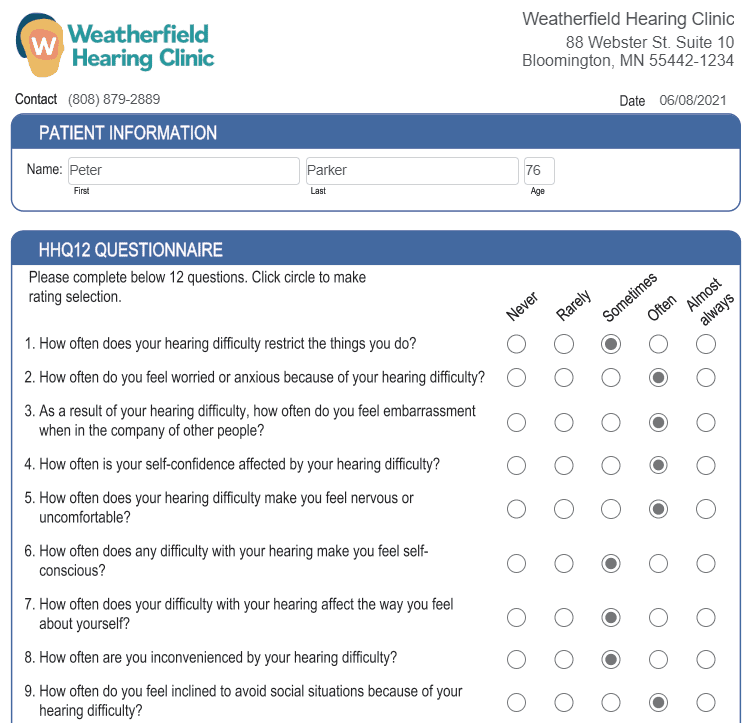
- Speech, spatial, and qualities of hearing scale survey (SSQ12)*
- Hearing handicap questionnaire (HHQ12)**
- Hearing inventory screening questionnaire
Patient surveys can be completed by the patient electronically from the comfort of their home or on a tablet in your waiting room. Survey responses will be automatically saved to the patient’s file in Blueprint OMS.
Additional survey tools at your fingertips!
Ida Institute’s survey tools allow your patients to provide you with valuable information about their hearing loss and motivation level prior to their appointment. These colorful, interactive surveys are designed to help patients describe daily communication situations.
Easily send these surveys directly from Blueprint OMS to your patients for electronic completion.
*William Noble, Niels Søgaard Jensen, Graham Naylor, Navjot Bhullar & Michael A. Akeroyd (2013) A short form of the Speech, Spatial and Qualities of Hearing scale suitable for clinical use: The SSQ12, International Journal of Audiology, 52:6, 409-412, DOI: 10.3109/14992027.2013.781278
**Stuart Gatehouse & William Noble (2004) The Speech, Spatial and Qualities of Hearing Scale (SSQ), International Journal of Audiology, 43:2, 85-99, DOI: 10.1080/14992020400050014
For more information, please contact us: here.
Related Articles
New Features and Enhancements in v4.7.0

In this article: New Features and Enhancements: Claims Tracking (US only) Send emails from a shared clinic email...
Read More3 NEW Seasonal templates now available in Blueprint OMS!

We have collaborated with Oticon to bring you THREE new seasonal email marketing templates! ...
Read More5 Benefits of Marketing Automation through Blueprint OMS
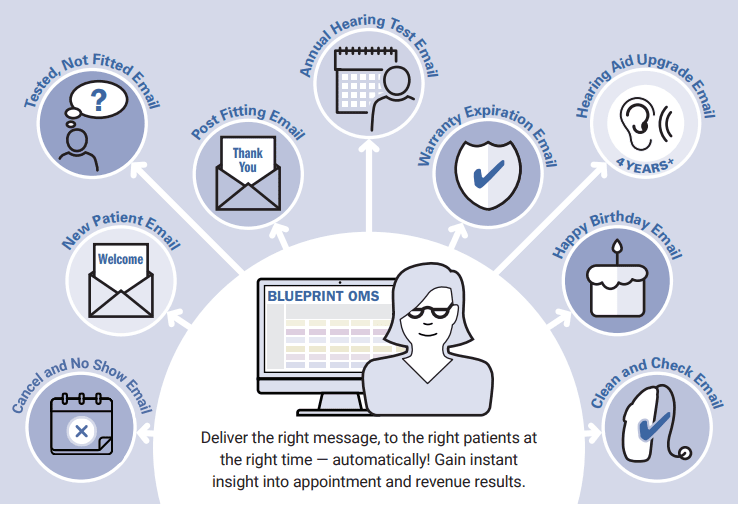
Why Blueprint OMS? Blueprint OMS is a comprehensive practice management software tailored specifically for audiology clinics. Among our...
Read More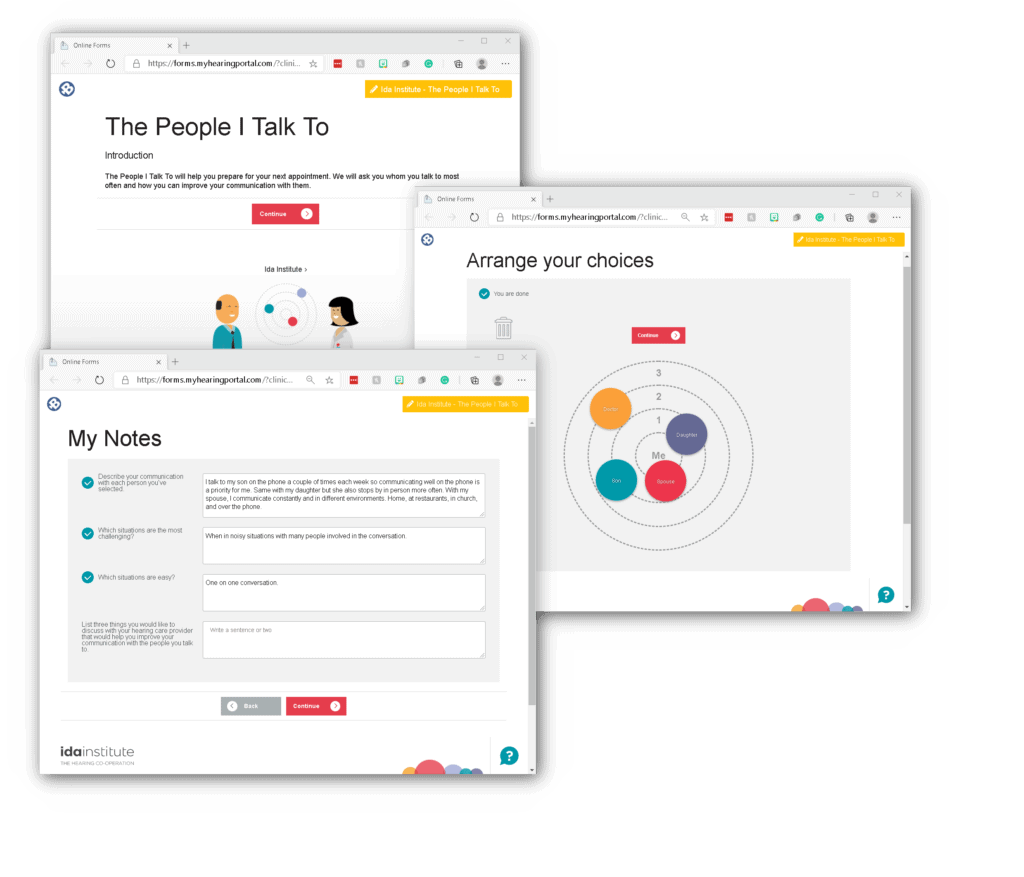
No comments